Everyone knows that, pencil is use for writing, but do you know it has a unique skill – to be able open the rust lock. Put some powder of pencil into key’s hole, and then you can open the rust lock. Do you know why pencil has this unique skill? It is because, pencil lead contain graphite. Graphite has the lubricating ability, so it can lubricate the rust lock.
Everyone knows that, pencil is use for writing, but do you know it has a unique skill – to be able open the rust lock. Put some powder of pencil into key’s hole, and then you can open the rust lock. Do you know why pencil has this unique skill? It is because, pencil lead contain graphite. Graphite has the lubricating ability, so it can lubricate the rust lock.
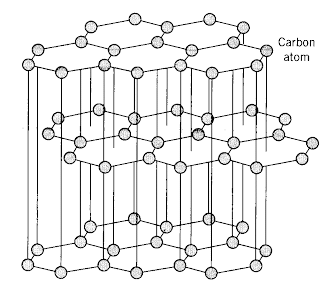
Graphite is one of the allotropes of carbon, located at the periodic table second period IVA race. The carbon is one kind of very common element, it widely exists by many kinds of forms in the atmosphere and the earth's crust. The carbon simple substance understands and use early by the person. Carbon is among ingredient of pig iron, wrought iron and steel.
There are three principal types of natural graphite, each occurring in different types of ore deposit:
(1) Crystalline flake graphite (or flake graphite for short) occurs as isolated, flat, plate-like particles with hexagonal edges if unbroken and when broken the edges can be irregular or angular
(2) Amorphous graphite occurs as fine particles and is the result of thermal metamorphism of coal, the last stage of coalification, and is sometimes called meta-anthracite. Very fine flake graphite is sometimes called amorphous in the trade
(3) Lump graphite (also called vein graphite) occurs in fissure veins or fractures and appears as massive platy intergrowths of fibrous or acicular crystalline aggregates, and is probably hydrothermal in origin.
☆Thermostable: Graphite melting point for 3850±50℃, boiling point for 4250℃, even if after the super high temperature electric arc ignition, the weight loss is very small, the thermal-expansion coefficient is also very small. The graphite intensity enhances along with the temperature strengthens, when 2000℃, the graphite intensity enhances one time.
★Electric conduction, thermal conductivity: The electric conductivity of graphite is 100 times stronger than other non-metallic ore. The thermal conductivity reduces along with temperature increment, even under the extremely high temperature, the graphite becomes the thermal insulation.
☆Lubricating ability: The graphite lubricity is decided by the graphite lamella's size, the lamella is bigger, then the friction coefficient is smaller, and the lubricity is better.
★Chemical stability: The graphite has the good chemical stability under the normal temperature, acidproof, bear the alkali and the organic corrosion solvent.
☆Plasticity: Graphite toughness is good, may grind the very thin slice